ASCLERA Injection
Sclerotherapy using Asclera Injections

Asclera is specifically designed for use on varicose veins of 1-3 mm in diameter. It’s also highly effective at treating veins measuring 1 mm or less, e.g. spider veins.
How does Asclera Injection work?
Like it’s many counterparts polidocanol is a sclerosing agent. This means that it causes severe irritation of the interior vein walls when injected. This reaction causes the vein to close down entirely, and it is then reabsorbed into the body. After several weeks it’s as if the treated vein were never there.
Which regions can be treated with Asclera?
In theory Asclera Injection can be used to permanently eliminate small veins anywhere on the body. In actual practice it’s used almost exclusively to treat veins of the lower extremities. This includes the thighs, legs, and ankles. Note that Asclera is not indicated for the treatment of varicose or spider veins of the feet.
Why is Asclera Injection superior to other medications of its kind?
Polidocanol is arguably the most trusted sclerosing agent in use today. This medication has been used in European countries for decades. It’s proven itself safe and effective on literally millions of patients. Asclera also has a mild anesthetic effect and is perhaps the most comfortable sclerosing medications in use today.
Who is a good candidate for sclerotherapy using Asclera?
Almost all adult male and female patients with smaller varicose veins or spider veins can benefit. This being said, those with various vascular conditions aren’t good candidates for Asclera (or any other sclerant medication).
Polidocanol may negatively interact with some prescription medications, most notably blood thinners. Before receiving sclerotherapy it’s important to make your doctor aware of all medications you are currently taking.
It is unknown at this time if Asclera Injection can be safely used on pregnant women. As such, if you’re pregnant, or think you may be pregnant, it’s best to postpone treatment.
Finally, Asclera or its ingredients may cause an allergic reaction in susceptible individuals. This is usually minor and resolves on its own.
What is involved in sclerotherapy using Asclera?
First, your vein specialist will clean the area that’s going to be treated with the Asclera Injection. For particularly pain-sensitive patients a topical numbing medication is often applied to the skin. This can also prove helpful if very large areas are being treated.
Using a small gauge needle tiny amounts of Asclera Injection medication are injected directly into the unwanted veins. At this point the site will be dressed exactly as it is following any other injection. For the vast majority of patients the total appointment time is less than ½ hour.
Is there a recovery period?
Some redness and bruising can be expected around the Asclera injection site but no recovery time, e.g. downtime, is involved. In cases where larger varicose leg veins are being treated patients may need to wear compression stockings. Even so, patients can return to normal activities immediately unless directed otherwise by the treating physician.
What are the potential side effects of Asclera injection?
Asclera (polidocanol) injection is used to treat varicose veins and spider veins. Like any medication or medical procedure, it can have potential side effects. Common side effects of Asclera injection may include:
- Mild discomfort or pain at the injection site.
- Bruising or redness around the treated area.
- Swelling or inflammation.
- Skin discoloration at the injection site.
Less common but more serious side effects may include:
- Allergic reactions, such as hives, itching, or rash.
- Ulceration or tissue damage at the injection site.
- Blood clot formation.
- Formation of small blood vessels or tiny red or purple spots.
- Skin injury or necrosis (rare).
What should I do before an Asclera injection session?
Before an Asclera injection session, follow these steps:
- Consultation: Schedule a consultation with a qualified healthcare provider to discuss your condition and treatment options.
- Medical History: Provide a complete medical history, including any allergies or medications you are taking.
- Physical Examination: Expect a physical examination to assess your veins and determine if Asclera is suitable for your condition.
- Dress Comfortably: Wear loose, comfortable clothing to the session, as you may need to expose the treatment area.
- Hydration: Stay well-hydrated in the days leading up to the injection session.
- Avoid Certain Medications: Your provider may advise you to avoid specific medications like blood thinners prior to the procedure.
- Questions: Prepare any questions you have about the treatment or aftercare.
Can patients resume regular activities immediately after the procedure, or are there restrictions?
After receiving Asclera injections for varicose veins or spider veins, patients can typically resume regular activities immediately. However, it’s advisable to avoid strenuous exercise, hot baths, and prolonged periods of standing for a day or two after the procedure. Patients are also encouraged to wear compression stockings and engage in regular walking, which promotes blood circulation in the treated area. It’s essential to follow the specific post-procedure instructions provided by the healthcare provider, as individual cases may vary. These instructions are designed to optimize the results and minimize any potential discomfort or complications.
Are there any lifestyle changes or precautions patients should consider post-treatment to prevent vein issues from recurring?
Certainly, after receiving treatment for varicose veins or spider veins, patients can take certain lifestyle measures and precautions to prevent vein issues from recurring:
- Regular Exercise: Engage in regular physical activity, such as walking or swimming, to improve circulation and strengthen leg muscles.
- Maintain a Healthy Weight: Maintain a healthy weight to reduce pressure on the veins and lower the risk of vein issues.
- Avoid Prolonged Standing or Sitting: Take breaks if your job requires long periods of standing or sitting. Stretch and move your legs regularly.
- Elevate Legs: Elevate your legs when resting to reduce swelling and improve blood flow back to the heart.
- Compression Stockings: Wear compression stockings as recommended by your healthcare provider to support blood circulation.
- Healthy Diet: Follow a balanced diet rich in fiber, low in salt, and high in fruits and vegetables to promote overall cardiovascular health.
- Avoid Tight Clothing: Avoid wearing tight clothes, especially around the waist and groin area, as they can restrict blood flow.
- Quit Smoking: If you smoke, consider quitting. Smoking can impair blood circulation and overall cardiovascular health.
Are the results of Asclera treatment permanent?
The results of Asclera treatment for varicose veins and spider veins are typically not considered permanent. While Asclera can effectively reduce the appearance of these veins, it does not address the underlying causes that lead to their development. Over time, new veins may form, and additional treatments may be needed to maintain the desired cosmetic results. Lifestyle factors, genetics, and other variables can also influence vein recurrence.
Will There Be Any Downtime After An Asclera Injection?
Luckily, there’s little to no downtime after you receive an Asclera injection, most people can easily continue their normal activities after the procedure! However, note that you may experience some degree of side effects which can include redness, itching, bruising, at the injection site which should resolve within few days. You will be advised to wear compression stockings for a short amount of time after the treatment to improve circulations and reduce swelling. Also, make sure you avoid hot baths, intense exercises, or even direct exposure to the sun for few days after the procedure to make sure you get the best results.
Are Asclera Injections Painful?
If you are afraid of pain through injections, then you don’t need to be worried since Asclera injections are generally well-tolerated, but there will be mild discomfort during the procedure. It’s because the needle used for this injection is small, so it can cause a small sting like feeling once the medication is injected into the affected veins. Discomfort will be brief and mild, so be not afraid. In some cases there can be swelling or redness, but it’s not that uncommon! Doctor will apply topical or numbing cream or even ice to minimize discomfort during the procedure, and compared to all the other invasive treatments, this is really minimal.
How Long Asclera Injection Results Will Last?
The results of Asclera injections can last pretty long, but it can vary from person to person at the same time. The treated veins will start disappearing within few weeks or months of the treatment. And the effects are permanent, which means the treated veins can never cause problems ever again as they are absorbed by the body and your blood has started flowing through other healthy veins.
What type of results can I expect?
Asclera and other sclerotherapy medications are well tolerated by most patients and the final results are excellent. Results aren’t immediately apparent, but in the weeks following sclerotherapy the treated veins are gradually reabsorbed by the body. Once this process is complete the veins in question disappears completely. While it’s true that other veins may appear in the future the treated veins are permanently gone.
Sclerotherapy: What to Expect
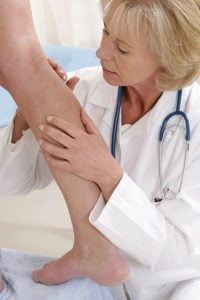
Depending on how far below your skin the damaged vein is, your doctor may use an ultrasound as part of the procedure.
I had been experiencing a deep pain in my left leg for several weeks. After several different appointments, a friend of mine recommended The Advanced Vein Center. They used an ultrasound to discover that a deep varicose vein was the cause. Later that week my leg was treated and the pain has gone away.
-Natalie (age 57)
Treatment
Before the injections, we will start by cleaning the skin around the veins to be treated. Then, a fine needle will be used to deliver a sclerosing agent into the damaged vein. Not all sclerosants are the same. Some solutions are liquid while others are a foam. All of them typically include:
- hypertonic saline solutions,
- polidocanol and
- sodium tetradecyl sulfate.
The sclerosing agent will cause the walls of the treated vein to collapse. The blood gets redirected into neighboring healthy veins. At this point your body’s natural metabolic process takes over. The dead vein is absorbed and eventually disappears. The result is that the offending vein is no longer visible and any discomfort that it was causing will vanish.
Smaller veins can be treated in a single session. Larger veins may require up to four (4) treatments.
Preparing for Sclerotherapy
The first step is a risk-free consultation with our office. Our knowledgeable and courteous staff will help you determine which procedure is the best given your individual situation. A personal medical history and physical exam will be taken in order to achieve this.
There are some medications that should be avoided starting a few days prior to the procedure. Don’t worry, we’ll remind you. Some pain medications such as Advil (ibuprofen) and Bufferin (aspirin) can increase your risk of bruising.
It is important to let us know about any medications that you are taking or if you have any other medical conditions prior to your procedure.
Side Effects and Risks of Sclerotherapy
There can be some minor, non-life-threatening side effects of the procedure. Thankfully, most sclerotherapy sessions are painless but you may feel some minor cramping or pain in the treated vein. Those that experience discomfort, describe it as a slight stinging or burning sensation. This does not mean that anything is wrong. It is just the sclerosing agent going to work on the vein. The discomfort may be a bit worse if the sclerosing compound comes into contact with the surrounding tissues. Again, this is no cause for alarm and will dissipate quickly.
The most common side effects of sclerotherapy injections include:
bruising,
- discomfort,
- redness or welts around the injection sites,
- skin discoloration,
- stinging and
- swelling.
It’s been four weeks since my final treatment and I’m ecstatic with the results. I no longer have to worry about buying clothes that will cover up my spider veins.
-Cassie (age 34)
Are there risks involved with Asclera Injection?
Any type of injection carries the possibility of infection yet in reality this risk is negligible. When infections do occur they tend to be very minor and respond well to topical or oral antibiotics.
Also note that sclerotherapy injections can cause minor scarring around the treatment site. As with infection, the actual risk is involved extremely low and scarring almost never occurs.
Has Asclera been approved by the Food and Drug Administration (FDA)?
Yes. In 2010 Asclera was approved by the FDA for the treatment of spider veins and smaller varicose veins. This means that most leg veins with a diameter of 5 mm or less can be effectively treated.
Risks and potential complications
Sclerotherapy is exceptionally safe and effective. It is a medical treatment however, and always carries an element of risk. Also, as with any medical procedure, it may not work exactly as intended. The following is a list of risks, complications, and adverse effects associated with sclerotherapy.
- Many patients only require a single treatment to eliminate all of their unwanted veins. This being said it’s entirely possible that multiple sessions will be necessary. This is especially true if a large area is being treated.
- Patients may experience some discomfort during and after treatment. This usually amounts to little more than a mild burning sensation during treatment. Minor itching over the next 24 hours has also been reported. Over the counter pain relievers may be used to manage these symptoms but this usually isn’t necessary.
- It is recommended that patients wear prescription compression stockings for up to two weeks after treatment. This isn’t always necessary, and in actuality this period is usually less than one week. According to many patients this is the most bothersome part of the entire process.
Minor risks
- Stockings are essential if you take part in strenuous physical activity during the two weeks following sclerotherapy.
- Some patients experience an allergic reaction to the medication used during treatment. This is very rare, almost always minor, and typically resolves quickly.
- Mild bruising can be expected in the area of treatment. This resolves as quickly as any other minor bruise.
- A blood clot may develop at the injection sites. In almost all cases these are harmless and resolve without complications.
- Mild blistering over the treatment area is possible. This is self-limiting and will go away on its own.
- Temporary discoloration known as hyperpigmentation may occur around the injection sites. This is temporary except in all but extremely rare cases.
- A network of small reddish or purplish vessels may form over the treatment area. This is known as “matte telangiectasias” and typically goes away without treatment in a matter of weeks.
- All injections carry a very small risk of infection. In almost all cases this is easily treated with topical or oral antibiotics.
Sclerotherapy with Asclera injections
—depending on the severity of your condition you may need 2 or more Asclera injections sessions.
If several sessions of asclera injections are required for optimal results it’s essential to schedule them at least 2 months apart. Only when the veins in question have been adequately treated will final results be achieved. In many cases this means the complete physical absence of the treated spider veins.
Today nearly all varicose and spider vein treatments are minimally invasive. For the most part they’re performed in-office using only a local anesthetic. Many surgeons choose to use lidocaine, a short lived, fast-acting local anesthetic. Recovery times are extremely minimal, and there’s rarely any “absolute” downtime. This means that most normal activities such as light household chores and office work can be resume immediately.
 Sclero- less invasive
Sclero- less invasive
By comparison, sclerotherapy is even less than most modern vein procedures. The veins are being injected with a medication, not physically removed as with a microphlebectomy. This results in virtually no downtime whatsoever. Some minor bruising of the overlying skin is typical yet serious complications are almost nonexistent. Scarring, if present at all, tends to be very minor. When scarring does occur it’s usually light enough to fade completely in the months and years following the procedure.
Safe, permanent spider vein treatments Bridgewater
The technical medical term for spider veins is telangiectasias. They usually appear as blotchy networks of thin bluish, purplish, or reddish veins. When examined closely these numerous small veins have a spidery appearance. Others liken the appearance to that of a spider’s web. In either case the name fits the condition perfectly.
Spider veins are usually treated for cosmetic reasons, e.g. improving the appearance of the legs (or calves or ankles). Yet with this being said the same underlying vein disease causes both spider and varicose veins. This is why a significant minority of spider vein patients also suffer from physical symptoms. The most common are pain, discomfort, tiredness, and swelling of one or both legs.
Symptoms
Other possible symptoms are itching, cramping, and less commonly Restless Leg Syndrome (RLS). Skin changes such as a reddish or brownish discoloration are also relatively common. In some cases these changes are permanent without the proper treatment.
Sclerotherapy Testimonial
After the Rvt took my medical history , I changed into a gown so she could perform the Doppler test on my legs and take some digital pictures to confirm medical necessity. She measured the veins both in the front and in the back of my legs where the main veins of the legs run. I was amazed that I could actually hear the difference between good and bad valves when she showed me that with the Venous Doppler . After my consultation with Dr Kavic, he explained that my veins weren’t in that bad shape, so we decided sclerotherapy was the best treatment plan for me. We set up my next appointment. It was much easier than I expected. I’m extremely happy with the results.
-Helen (age 59)
Important Safety Information
Indication:
Asclera® (polidocanol) Injection is a prescription medicine that is used in a procedure called sclerotherapy to remove unwanted veins on your legs. It is administered by a healthcare provider to treat two types of veins:
Uncomplicated spider veins (very small varicose veins ≤ 1 mm in diameter)
Uncomplicated small varicose veins (1 to 3 mm in diameter) known as reticular veins
Asclera® has not been studied in varicose veins more than 3 mm in diameter.
IMPORTANT SAFETY INFORMATION FOR PATIENTS:
For intravenous use only.
CONTRAINDICATIONS: Asclera® (polidocanol) Injection is contraindicated for patients with known allergy (anaphylaxis) to polidocanol and patients with acute vein and blood clotting diseases.
WARNINGS AND PRECAUTIONS:
Anaphylaxis: Severe allergic reactions have been reported following polidocanol use, including anaphylactic reactions, some of them fatal. Severe reactions are most frequent with use of larger volumes (> 3 mL). The dose of polidocanol should therefore be minimized. Please notify your healthcare provider if you have a known history of severe allergies or allergy to polidocanol.
Venous Thrombosis and Pulmonary Embolism: Asclera can cause venous thrombosis and subsequent pulmonary embolism or other thrombotic events. Your physician should follow administration instructions closely and monitor for signs of venous thrombosis after treatment. Patients with reduced mobility, history of deep vein thrombosis or pulmonary embolism, or recent (within 3 months) major surgery, prolonged hospitalization or pregnancy are at increased risk for developing thrombosis.
Arterial Embolism: Stroke, transient ischemic attack, myocardial infarction, and impaired cardiac function have been reported in close temporal relationship with polidocanol administration. These events may be caused by air embolism when using the product foamed with room air (high nitrogen concentration) or thromboembolism. The safety and efficacy of polidocanol foamed with room air has not been established and its use should be avoided.
Accidental injection into an artery can cause severe necrosis, ischemia or gangrene.
Care should be taken in intravenous needle placement and the smallest effective volume at each injection site should be used. If intra-arterial injection of polidocanol occurs, consult a vascular surgeon immediately.
After the injection session is completed, apply compression with a stocking or bandage, and walk for 15-20 minutes. Your healthcare provider will provide monitoring during this period to treat any possible anaphylactic or allergic reactions.
Maintain compression for 2 to 3 days after treatment of spider veins and for 5 to 7 days for reticular veins, or as directed by your Healthcare Provider. For extensive varicosities, longer compression treatment with compression bandages or a gradient compression stocking of a higher compression class is recommended. Post-treatment compression is necessary to reduce the risk of deep vein thrombosis.
ADVERSE REACTIONS: In clinical studies, the following adverse reactions were observed after using Asclera® and were more common with Asclera® than placebo: injection site hematoma, injection site irritation, injection site discoloration, injection site pain, injection site itching, injection site warmth, neovascularization, injection site clotting.
You are encouraged to report any suspected adverse events. To report SUSPECTED ADVERSE REACTIONS, contact your Healthcare Provider, Merz North America at 1-866-862-1211, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Asclera® (polidocanol) Injection
Distributed by Merz North America

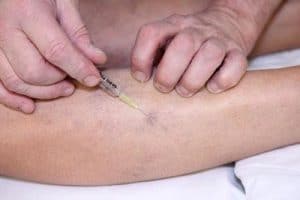

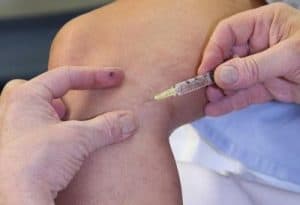 bruising,
bruising,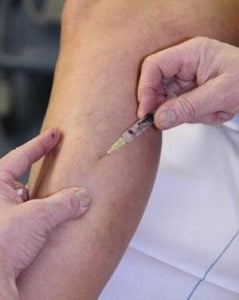 Sclero- less invasive
Sclero- less invasive